Charcot beschreef ALS voor het eerst tussen 1865 en 1874 als een ziekte die volwassenen treft en ontstaat door een onbekende, voortschrijdende aftakeling van het zenuwstelsel dat onze spieren aanstuurt. Dit leidt snel tot ernstige spierzwakte en afbraak van spieren. Er is geen genezing voor ALS en geen bewezen behandeling om het te voorkomen of terug te draaien. Er zijn wel twee medicijnen, riluzol en edaravone, specifiek goedgekeurd voor de behandeling van ALS, en veel andere worden nog getest of zijn in klinische proeven. Tot nu toe is er nog geen echte doorbraak in de behandeling van deze slopende ziekte. Ons begrip van ALS en hoe het ontstaat, wordt echter steeds beter. We weten dat schade door zuurstof (oxidatieve stress), veranderingen in de stofwisseling die afhankelijk zijn van NAD+ en de toestand van redox, en afwijkende werking en dynamiek van de mitochondriën, de energiefabriekjes in onze zenuwcellen, een belangrijke rol spelen. Daarom worden verschillende antioxidanten en stoffen die NAD+ aanmaken voorgesteld als mogelijke behandelingen voor ALS. Deze review bekijkt niet alleen het gebruik van deze stoffen op zichzelf, maar ook de mogelijkheid om ze te combineren, en zelfs als onderdeel van bredere, gecombineerde therapieën.
Artikel
NAD+ Precursors and Antioxidants for the Treatment of Amyotrophic Lateral Sclerosis
Referenties
1. Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., Shaw P.J., Simmons Z., van den Berg L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primers. 2017;3:1–19. doi: 10.1038/nrdp.2017.71. [PubMed] [CrossRef] [Google Scholar]
2. Masrori P., Van Damme P. Amyotrophic Lateral Sclerosis: A Clinical Review. Eur. J. Neurol. 2020;27:1918–1929. doi: 10.1111/ene.14393. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
3. Trojsi F., D’Alvano G., Bonavita S., Tedeschi G. Genetics and Sex in the Pathogenesis of Amyotrophic Lateral Sclerosis (ALS): Is There a Link? Int. J. Mol. Sci. 2020;21:3647. doi: 10.3390/ijms21103647. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
4. Chiò A., Moglia C., Canosa A., Manera U., D’Ovidio F., Vasta R., Grassano M., Brunetti M., Barberis M., Corrado L., et al. ALS Phenotype Is Influenced by Age, Sex, and Genetics: A Population-Based Study. Neurology. 2020;94:e802–e810. doi: 10.1212/WNL.0000000000008869. [PubMed] [CrossRef] [Google Scholar]
5. Mehta P.R., Jones A.R., Opie-Martin S., Shatunov A., Iacoangeli A., Al Khleifat A., Smith B.N., Topp S., Morrison K.E., Shaw P.J., et al. Younger Age of Onset in Familial Amyotrophic Lateral Sclerosis Is a Result of Pathogenic Gene Variants, Rather than Ascertainment Bias. J. Neurol. Neurosurg. Psychiatry. 2019;90:268–271. doi: 10.1136/jnnp-2018-319089. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
6. McCombe P.A., Garton F.C., Katz M., Wray N.R., Henderson R.D. What Do We Know about the Variability in Survival of Patients with Amyotrophic Lateral Sclerosis? Expert Rev. Neurother. 2020;20:921–941. doi: 10.1080/14737175.2020.1785873. [PubMed] [CrossRef] [Google Scholar]
7. Kiernan M.C., Vucic S., Talbot K., McDermott C.J., Hardiman O., Shefner J.M., Al-Chalabi A., Huynh W., Cudkowicz M., Talman P., et al. Improving Clinical Trial Outcomes in Amyotrophic Lateral Sclerosis. Nat. Rev. Neurol. 2021;17:104–118. doi: 10.1038/s41582-020-00434-z. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
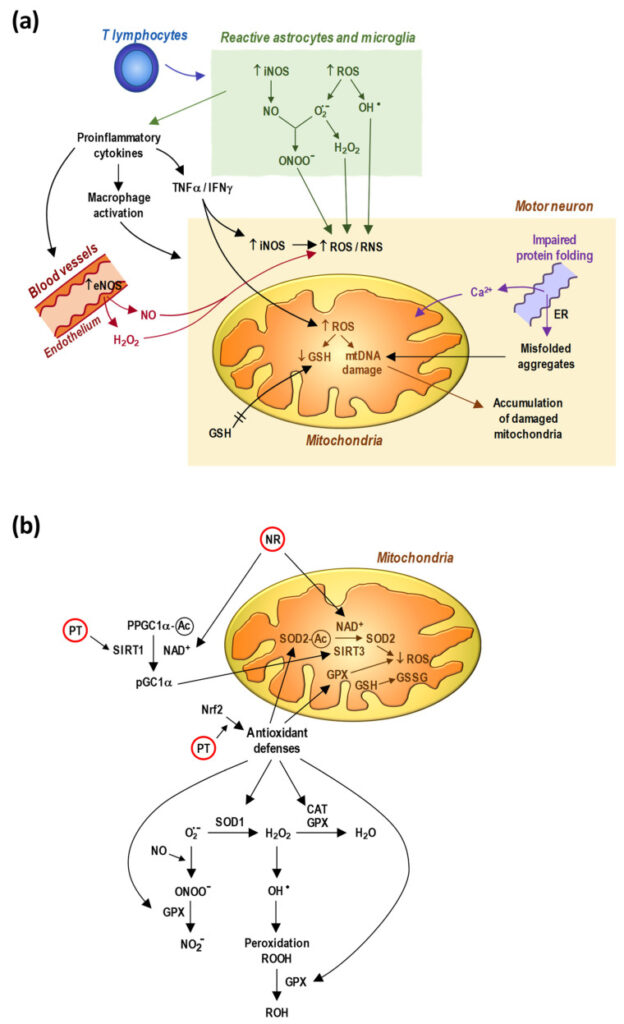
8. Obrador E., Salvador-Palmer R., López-Blanch R., Jihad-Jebbar A., Vallés S.L., Estrela J.M. The Link between Oxidative Stress, Redox Status, Bioenergetics and Mitochondria in the Pathophysiology of ALS. Int. J. Mol. Sci. 2021;22:6352. doi: 10.3390/ijms22126352. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
9. Sies H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants. 2020;9:852. doi: 10.3390/antiox9090852. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
10. Carretero J., Obrador E., Esteve J.M., Ortega A., Pellicer J.A., Sempere F.V., Estrela J.M. Tumoricidal Activity of Endothelial Cells. Inhibition of Endothelial Nitric Oxide Production Abrogates Tumor Cytotoxicity Induced by Hepatic Sinusoidal Endothelium in Response to B16 Melanoma Adhesion in Vitro. J. Biol. Chem. 2001;276:25775–25782. doi: 10.1074/jbc.M101148200. [PubMed] [CrossRef] [Google Scholar]
11. Drechsel D.A., Estévez A.G., Barbeito L., Beckman J.S. Nitric Oxide-Mediated Oxidative Damage and the Progressive Demise of Motor Neurons in ALS. Neurotox. Res. 2012;22:251–264. doi: 10.1007/s12640-012-9322-y. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
12. Cunha-Oliveira T., Montezinho L., Mendes C., Firuzi O., Saso L., Oliveira P.J., Silva F.S.G. Oxidative Stress in Amyotrophic Lateral Sclerosis: Pathophysiology and Opportunities for Pharmacological Intervention. Oxid. Med. Cell. Longev. 2020;2020:5021694. doi: 10.1155/2020/5021694. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
13. Silva J.M., Nobre M.S.C., Albino S.L., Lócio L.L., Nascimento A.P.S., Scotti L., Scotti M.T., Oshiro-Junior J.A., Lima M.C.A., Mendonça-Junior F.J.B., et al. Secondary Metabolites with Antioxidant Activities for the Putative Treatment of Amyotrophic Lateral Sclerosis (ALS): “Experimental Evidences” Oxid. Med. Cell. Longev. 2020;2020:5642029. doi: 10.1155/2020/5642029. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
14. Griffiths H.B.S., Williams C., King S.J., Allison S.J. Nicotinamide Adenine Dinucleotide (NAD+): Essential Redox Metabolite, Co-Substrate and an Anti-Cancer and Anti-Ageing Therapeutic Target. Biochem. Soc. Trans. 2020;48:733–744. doi: 10.1042/BST20190033. [PubMed] [CrossRef] [Google Scholar]
15. Gilmour B.C., Gudmundsrud R., Frank J., Hov A., Lautrup S., Aman Y., Røsjø H., Brenner C., Ziegler M., Tysnes O.-B., et al. Targeting NAD+ in Translational Research to Relieve Diseases and Conditions of Metabolic Stress and Ageing. Mech. Ageing Dev. 2020;186:111208. doi: 10.1016/j.mad.2020.111208. [PubMed] [CrossRef] [Google Scholar]
16. Li F., Chong Z.Z., Maiese K. Navigating Novel Mechanisms of Cellular Plasticity with the NAD+ Precursor and Nutrient Nicotinamide. Front. Biosci. 2004;9:2500–2520. doi: 10.2741/1412. [PubMed] [CrossRef] [Google Scholar]
17. Lautrup S., Sinclair D.A., Mattson M.P., Fang E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019;30:630–655. doi: 10.1016/j.cmet.2019.09.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
18. Yang T., Sauve A.A. NAD Metabolism and Sirtuins: Metabolic Regulation of Protein Deacetylation in Stress and Toxicity. AAPS J. 2006;8:E632–E643. doi: 10.1208/aapsj080472. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. Wang X., Hu X., Zhang L., Xu X., Sakurai T. Nicotinamide Mononucleotide Administration after Sever Hypoglycemia Improves Neuronal Survival and Cognitive Function in Rats. Brain. Res. Bull. 2020;160:98–106. doi: 10.1016/j.brainresbull.2020.04.022. [PubMed] [CrossRef] [Google Scholar]
20. Xie N., Zhang L., Gao W., Huang C., Huber P.E., Zhou X., Li C., Shen G., Zou B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal. Transduct. Target. Ther. 2020;5:227. doi: 10.1038/s41392-020-00311-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
21. Roboon J., Hattori T., Ishii H., Takarada-Iemata M., Nguyen D.T., Heer C.D., O’Meally D., Brenner C., Yamamoto Y., Okamoto H., et al. Inhibition of CD38 and Supplementation of Nicotinamide Riboside Ameliorate Lipopolysaccharide-Induced Microglial and Astrocytic Neuroinflammation by Increasing NAD. J. Neurochem. 2021;158:311–327. doi: 10.1111/jnc.15367. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
22. Pehar M., Harlan B.A., Killoy K.M., Vargas M.R. Nicotinamide Adenine Dinucleotide Metabolism and Neurodegeneration. Antioxid. Redox Signal. 2018;28:1652–1668. doi: 10.1089/ars.2017.7145. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
23. Liu D., Gharavi R., Pitta M., Gleichmann M., Mattson M.P. Nicotinamide Prevents NAD+ Depletion and Protects Neurons against Excitotoxicity and Cerebral Ischemia: NAD+ Consumption by SIRT1 May Endanger Energetically Compromised Neurons. Neuromol. Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
24. Bouchard V.J., Rouleau M., Poirier G.G. PARP-1, a Determinant of Cell Survival in Response to DNA Damage. Exp. Hematol. 2003;31:446–454. doi: 10.1016/S0301-472X(03)00083-3. [PubMed] [CrossRef] [Google Scholar]
25. Rajamohan S.B., Pillai V.B., Gupta M., Sundaresan N.R., Birukov K.G., Samant S., Hottiger M.O., Gupta M.P. SIRT1 Promotes Cell Survival under Stress by Deacetylation-Dependent Deactivation of Poly(ADP-Ribose) Polymerase 1. Mol. Cell. Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
26. Chong Z.-Z., Lin S.-H., Li F., Maiese K. The Sirtuin Inhibitor Nicotinamide Enhances Neuronal Cell Survival during Acute Anoxic Injury through AKT, BAD, PARP, and Mitochondrial Associated “Anti-Apoptotic” Pathways. Curr. Neurovasc. Res. 2005;2:271–285. doi: 10.2174/156720205774322584. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
27. Li Y., Xu W., McBurney M.W., Longo V.D. SirT1 Inhibition Reduces IGF-I/IRS-2/Ras/ERK1/2 Signaling and Protects Neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
28. Katsyuba E., Romani M., Hofer D., Auwerx J. NAD+ Homeostasis in Health and Disease. Nat. Metab. 2020;2:9–31. doi: 10.1038/s42255-019-0161-5. [PubMed] [CrossRef] [Google Scholar]
29. Belenky P., Bogan K.L., Brenner C. NAD+ Metabolism in Health and Disease. Trends. Biochem. Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [PubMed] [CrossRef] [Google Scholar]
30. Strømland Ø., Niere M., Nikiforov A.A., VanLinden M.R., Heiland I., Ziegler M. Keeping the Balance in NAD Metabolism. Biochem. Soc. Trans. 2019;47:119–130. doi: 10.1042/BST20180417. [PubMed] [CrossRef] [Google Scholar]
31. Slomka M., Zieminska E., Salinska E., Lazarewicz J.W. Neuroprotective Effects of Nicotinamide and 1-Methylnicotinamide in Acute Excitotoxicity in Vitro. Folia Neuropathol. 2008;46:69–80. [PubMed] [Google Scholar]
32. Sasaki Y., Araki T., Milbrandt J. Stimulation of Nicotinamide Adenine Dinucleotide Biosynthetic Pathways Delays Axonal Degeneration after Axotomy. J. Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
33. Schöndorf D.C., Ivanyuk D., Baden P., Sanchez-Martinez A., De Cicco S., Yu C., Giunta I., Schwarz L.K., Di Napoli G., Panagiotakopoulou V., et al. The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in IPSC and Fly Models of Parkinson’s Disease. Cell Rep. 2018;23:2976–2988. doi: 10.1016/j.celrep.2018.05.009. [PubMed] [CrossRef] [Google Scholar]
34. Harlan B.A., Pehar M., Sharma D.R., Beeson G., Beeson C.C., Vargas M.R. Enhancing NAD+ Salvage Pathway Reverts the Toxicity of Primary Astrocytes Expressing Amyotrophic Lateral Sclerosis-Linked Mutant Superoxide Dismutase 1 (SOD1) J. Biol. Chem. 2016;291:10836–10846. doi: 10.1074/jbc.M115.698779. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
35. Gerdts J., Brace E.J., Sasaki Y., DiAntonio A., Milbrandt J. SARM1 Activation Triggers Axon Degeneration Locally via NAD+ Destruction. Science. 2015;348:453–457. doi: 10.1126/science.1258366. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
36. Alano C.C., Tran A., Tao R., Ying W., Karliner J.S., Swanson R.A. Differences among Cell Types in NAD(+) Compartmentalization: A Comparison of Neurons, Astrocytes, and Cardiac Myocytes. J. Neurosci. Res. 2007;85:3378–3385. doi: 10.1002/jnr.21479. [PubMed] [CrossRef] [Google Scholar]
37. Harlan B.A., Killoy K.M., Pehar M., Liu L., Auwerx J., Vargas M.R. Evaluation of the NAD+ Biosynthetic Pathway in ALS Patients and Effect of Modulating NAD+ Levels in HSOD1-Linked ALS Mouse Models. Exp. Neurol. 2020;327:113219. doi: 10.1016/j.expneurol.2020.113219. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
38. Park J.H., Long A., Owens K., Kristian T. Nicotinamide Mononucleotide Inhibits Post-Ischemic NAD(+) Degradation and Dramatically Ameliorates Brain Damage Following Global Cerebral Ischemia. Neurobiol. Dis. 2016;95:102–110. doi: 10.1016/j.nbd.2016.07.018. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
39. Karnati S., Lüers G., Pfreimer S., Baumgart-Vogt E. Mammalian SOD2 Is Exclusively Located in Mitochondria and Not Present in Peroxisomes. Histochem. Cell. Biol. 2013;140:105–117. doi: 10.1007/s00418-013-1099-4. [PubMed] [CrossRef] [Google Scholar]
40. Jo S.H., Son M.K., Koh H.J., Lee S.M., Song I.H., Kim Y.O., Lee Y.S., Jeong K.S., Kim W.B., Park J.W., et al. Control of Mitochondrial Redox Balance and Cellular Defense against Oxidative Damage by Mitochondrial NADP+-Dependent Isocitrate Dehydrogenase. J. Biol. Chem. 2001;276:16168–16176. doi: 10.1074/jbc.M010120200. [PubMed] [CrossRef] [Google Scholar]
41. Sies H., Berndt C., Jones D.P. Oxidative Stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [PubMed] [CrossRef] [Google Scholar]
42. Mårtensson J., Lai J.C., Meister A. High-Affinity Transport of Glutathione Is Part of a Multicomponent System Essential for Mitochondrial Function. Proc. Natl. Acad. Sci. USA. 1990;87:7185–7189. doi: 10.1073/pnas.87.18.7185. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
43. Berman S.B., Watkins S.C., Hastings T.G. Quantitative Biochemical and Ultrastructural Comparison of Mitochondrial Permeability Transition in Isolated Brain and Liver Mitochondria: Evidence for Reduced Sensitivity of Brain Mitochondria. Exp. Neurol. 2000;164:415–425. doi: 10.1006/exnr.2000.7438. [PubMed] [CrossRef] [Google Scholar]
44. Jain A., Mårtensson J., Stole E., Auld P.A., Meister A. Glutathione Deficiency Leads to Mitochondrial Damage in Brain. Proc. Natl. Acad. Sci. USA. 1991;88:1913–1917. doi: 10.1073/pnas.88.5.1913. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
45. Killoy K.M., Harlan B.A., Pehar M., Helke K.L., Johnson J.A., Vargas M.R. Decreased Glutathione Levels Cause Overt Motor Neuron Degeneration in HSOD1WT Over-Expressing Mice. Exp. Neurol. 2018;302:129–135. doi: 10.1016/j.expneurol.2018.01.004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
46. Weiduschat N., Mao X., Hupf J., Armstrong N., Kang G., Lange D.J., Mitsumoto H., Shungu D.C. Motor Cortex Glutathione Deficit in ALS Measured in Vivo with the J-Editing Technique. Neurosci. Lett. 2014;570:102–107. doi: 10.1016/j.neulet.2014.04.020. [PubMed] [CrossRef] [Google Scholar]
47. Magni G., Amici A., Emanuelli M., Raffaelli N., Ruggieri S. Enzymology of NAD+ Synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1999;73:135–182. doi: 10.1002/9780470123195.ch5. xi. [PubMed] [CrossRef] [Google Scholar]
48. Bogan K.L., Brenner C. Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD+ Precursor Vitamins in Human Nutrition. Annu. Rev. Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [PubMed] [CrossRef] [Google Scholar]
49. Grozio A., Sociali G., Sturla L., Caffa I., Soncini D., Salis A., Raffaelli N., De Flora A., Nencioni A., Bruzzone S. CD73 Protein as a Source of Extracellular Precursors for Sustained NAD+ Biosynthesis in FK866-Treated Tumor Cells. J. Biol. Chem. 2013;288:25938–25949. doi: 10.1074/jbc.M113.470435. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
50. Braidy N., Berg J., Clement J., Khorshidi F., Poljak A., Jayasena T., Grant R., Sachdev P. Role of Nicotinamide Adenine Dinucleotide and Related Precursors as Therapeutic Targets for Age-Related Degenerative Diseases: Rationale, Biochemistry, Pharmacokinetics, and Outcomes. Antioxid. Redox Signal. 2019;30:251–294. doi: 10.1089/ars.2017.7269. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
51. Pirinen E., Auranen M., Khan N.A., Brilhante V., Urho N., Pessia A., Hakkarainen A., Kuula J., Heinonen U., Schmidt M.S., et al. Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020;31:1078–1090.e5. doi: 10.1016/j.cmet.2020.04.008. [PubMed] [CrossRef] [Google Scholar]
52. Habibe M.N., Kellar J.Z. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2021. Niacin Toxicity. [Google Scholar]
53. Hwang E.S., Song S.B. Possible Adverse Effects of High-Dose Nicotinamide: Mechanisms and Safety Assessment. Biomolecules. 2020;10:687. doi: 10.3390/biom10050687. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
54. Harrison I.F., Powell N.M., Dexter D.T. The Histone Deacetylase Inhibitor Nicotinamide Exacerbates Neurodegeneration in the Lactacystin Rat Model of Parkinson’s Disease. J. Neurochem. 2019;148:136–156. doi: 10.1111/jnc.14599. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
55. Ito T.K., Sato T., Hakamata A., Onoda Y., Sato S., Yamazaki F., Horikawa M., Takahashi Y., Kitamoto T., Suzuki M., et al. A Nonrandomized Study of Single Oral Supplementation within the Daily Tolerable Upper Level of Nicotinamide Affects Blood Nicotinamide and NAD+ Levels in Healthy Subjects. Transl. Med. Aging. 2020;4:45–54. doi: 10.1016/j.tma.2020.04.002. [CrossRef] [Google Scholar]
56. Olsson A., Olofsson T., Pero R.W. Specific Binding and Uptake of Extracellular Nicotinamide in Human Leukemic K-562 Cells. Biochem. Pharmacol. 1993;45:1191–1200. doi: 10.1016/0006-2952(93)90270-7. [PubMed] [CrossRef] [Google Scholar]
57. Avalos J.L., Bever K.M., Wolberger C. Mechanism of Sirtuin Inhibition by Nicotinamide: Altering the NAD(+) Cosubstrate Specificity of a Sir2 Enzyme. Mol. Cell. 2005;17:855–868. doi: 10.1016/j.molcel.2005.02.022. [PubMed] [CrossRef] [Google Scholar]
58. Campagna R., Mateuszuk Ł., Wojnar-Lason K., Kaczara P., Tworzydło A., Kij A., Bujok R., Mlynarski J., Wang Y., Sartini D., et al. Nicotinamide N-Methyltransferase in Endothelium Protects against Oxidant Stress-Induced Endothelial Injury. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868:119082. doi: 10.1016/j.bbamcr.2021.119082. [PubMed] [CrossRef] [Google Scholar]
59. Ratajczak J., Joffraud M., Trammell S.A.J., Ras R., Canela N., Boutant M., Kulkarni S.S., Rodrigues M., Redpath P., Migaud M.E., et al. NRK1 Controls Nicotinamide Mononucleotide and Nicotinamide Riboside Metabolism in Mammalian Cells. Nat. Commun. 2016;7:13103. doi: 10.1038/ncomms13103. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
60. Grozio A., Mills K.F., Yoshino J., Bruzzone S., Sociali G., Tokizane K., Lei H.C., Cunningham R., Sasaki Y., Migaud M.E., et al. Slc12a8 Is a Nicotinamide Mononucleotide Transporter. Nat. Metab. 2019;1:47–57. doi: 10.1038/s42255-018-0009-4. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
61. Yoshino M., Yoshino J., Kayser B.D., Patti G.J., Franczyk M.P., Mills K.F., Sindelar M., Pietka T., Patterson B.W., Imai S.-I., et al. Nicotinamide Mononucleotide Increases Muscle Insulin Sensitivity in Prediabetic Women. Science. 2021;372:1224–1229. doi: 10.1126/science.abe9985. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
62. Hara N., Yamada K., Shibata T., Osago H., Hashimoto T., Tsuchiya M. Elevation of Cellular NAD Levels by Nicotinic Acid and Involvement of Nicotinic Acid Phosphoribosyltransferase in Human Cells. J. Biol. Chem. 2007;282:24574–24582. doi: 10.1074/jbc.M610357200. [PubMed] [CrossRef] [Google Scholar]
63. Trammell S.A.J., Schmidt M.S., Weidemann B.J., Redpath P., Jaksch F., Dellinger R.W., Li Z., Abel E.D., Migaud M.E., Brenner C. Nicotinamide Riboside Is Uniquely and Orally Bioavailable in Mice and Humans. Nat. Commun. 2016;7:1–14. doi: 10.1038/ncomms12948. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
64. Dellinger R.W., Santos S.R., Morris M., Evans M., Alminana D., Guarente L., Marcotulli E. Repeat Dose NRPT (Nicotinamide Riboside and Pterostilbene) Increases NAD+ Levels in Humans Safely and Sustainably: A Randomized, Double-Blind, Placebo-Controlled Study. NPJ Aging Mech. Dis. 2017;3:17. doi: 10.1038/s41514-017-0016-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
65. Elhassan Y.S., Kluckova K., Fletcher R.S., Schmidt M.S., Garten A., Doig C.L., Cartwright D.M., Oakey L., Burley C.V., Jenkinson N., et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-Inflammatory Signatures. Cell Rep. 2019;28:1717–1728.e6. doi: 10.1016/j.celrep.2019.07.043. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
66. Conze D., Brenner C., Kruger C.L. Safety and Metabolism of Long-Term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Healthy Overweight Adults. Sci. Rep. 2019;9:9772. doi: 10.1038/s41598-019-46120-z. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
67. Christ W., Coper H. Preparation and Purification of Nicotinamide Mononucleotide Analogs. Methods Enzymol. 1980;66:71–81. doi: 10.1016/0076-6879(80)66440-4. [PubMed] [CrossRef] [Google Scholar]
68. Tarragó M.G., Chini C.C.S., Kanamori K.S., Warner G.M., Caride A., de Oliveira G.C., Rud M., Samani A., Hein K.Z., Huang R., et al. A Potent and Specific CD38 Inhibitor Ameliorates Age-Related Metabolic Dysfunction by Reversing Tissue NAD+ Decline. Cell Metab. 2018;27:1081–1095.e10. doi: 10.1016/j.cmet.2018.03.016. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
69. Bian C., Zhang C., Luo T., Vyas A., Chen S.-H., Liu C., Kassab M.A., Yang Y., Kong M., Yu X. NADP+ Is an Endogenous PARP Inhibitor in DNA Damage Response and Tumor Suppression. Nat. Commun. 2019;10:693. doi: 10.1038/s41467-019-08530-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
70. Hughes R.O., Bosanac T., Mao X., Engber T.M., DiAntonio A., Milbrandt J., Devraj R., Krauss R. Small Molecule SARM1 Inhibitors Recapitulate the SARM1-/- Phenotype and Allow Recovery of a Metastable Pool of Axons Fated to Degenerate. Cell Rep. 2021;34:108588. doi: 10.1016/j.celrep.2020.108588. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
71. Ito H., Wate R., Zhang J., Ohnishi S., Kaneko S., Ito H., Nakano S., Kusaka H. Treatment with Edaravone, Initiated at Symptom Onset, Slows Motor Decline and Decreases SOD1 Deposition in ALS Mice. Exp. Neurol. 2008;213:448–455. doi: 10.1016/j.expneurol.2008.07.017. [PubMed] [CrossRef] [Google Scholar]
72. Zona C., Pieri M., Carunchio I. Voltage-Dependent Sodium Channels in Spinal Cord Motor Neurons Display Rapid Recovery from Fast Inactivation in a Mouse Model of Amyotrophic Lateral Sclerosis. J. Neurophysiol. 2006;96:3314–3322. doi: 10.1152/jn.00566.2006. [PubMed] [CrossRef] [Google Scholar]
73. Luo L., Song Z., Li X., Huiwang, Zeng Y., Qinwang, Meiqi, He J. Efficacy and Safety of Edaravone in Treatment of Amyotrophic Lateral Sclerosis-a Systematic Review and Meta-Analysis. Neurol. Sci. 2019;40:235–241. doi: 10.1007/s10072-018-3653-2. [PubMed] [CrossRef] [Google Scholar]
74. Ortiz J.F., Khan S.A., Salem A., Lin Z., Iqbal Z., Jahan N. Post-Marketing Experience of Edaravone in Amyotrophic Lateral Sclerosis: A Clinical Perspective and Comparison With the Clinical Trials of the Drug. Cureus. 2020;12:e10818. doi: 10.7759/cureus.10818. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
75. Kwieciński H., Janik P., Jamrozik Z., Opuchlik A. The effect of selegiline and vitamin E in the treatment of ALS: An open randomized clinical trials. Neurol. Neurochir. Pol. 2001;35:101–106. [PubMed] [Google Scholar]
76. Desnuelle C., Dib M., Garrel C., Favier A. A Double-Blind, Placebo-Controlled Randomized Clinical Trial of Alpha-Tocopherol (Vitamin E) in the Treatment of Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Other Motor. Neuron Disord. 2001;2:9–18. doi: 10.1080/146608201300079364. [PubMed] [CrossRef] [Google Scholar]
77. Kaufmann P., Thompson J.L.P., Levy G., Buchsbaum R., Shefner J., Krivickas L.S., Katz J., Rollins Y., Barohn R.J., Jackson C.E., et al. Phase II Trial of CoQ10 for ALS Finds Insufficient Evidence to Justify Phase III. Ann. Neurol. 2009;66:235–244. doi: 10.1002/ana.21743. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
78. Nagase M., Yamamoto Y., Miyazaki Y., Yoshino H. Increased Oxidative Stress in Patients with Amyotrophic Lateral Sclerosis and the Effect of Edaravone Administration. Redox Rep. 2016;21:104–112. doi: 10.1179/1351000215Y.0000000026. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
79. Weishaupt J.H., Bartels C., Pölking E., Dietrich J., Rohde G., Poeggeler B., Mertens N., Sperling S., Bohn M., Hüther G., et al. Reduced Oxidative Damage in ALS by High-Dose Enteral Melatonin Treatment. J. Pineal. Res. 2006;41:313–323. doi: 10.1111/j.1600-079X.2006.00377.x. [PubMed] [CrossRef] [Google Scholar]
80. ALSUNTANGLED GROUP ALSUntangled #61: Melatonin. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2021:1–4. doi: 10.1080/21678421.2021.1927103. [PubMed] [CrossRef] [Google Scholar]
81. Fitzgerald K.C., O’Reilly É.J., Fondell E., Falcone G.J., McCullough M.L., Park Y., Kolonel L.N., Ascherio A. Intakes of Vitamin C and Carotenoids and Risk of Amyotrophic Lateral Sclerosis: Pooled Results from 5 Cohort Studies. Ann. Neurol. 2013;73:236–245. doi: 10.1002/ana.23820. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
82. Andreassen O.A., Dedeoglu A., Klivenyi P., Beal M.F., Bush A.I. N-Acetyl-L-Cysteine Improves Survival and Preserves Motor Performance in an Animal Model of Familial Amyotrophic Lateral Sclerosis. Neuroreport. 2000;11:2491–2493. doi: 10.1097/00001756-200008030-00029. [PubMed] [CrossRef] [Google Scholar]
83. Louwerse E.S., Weverling G.J., Bossuyt P.M.M., Meyjes F.E.P., Jong J.M.B.V. de Randomized, Double-Blind, Controlled Trial of Acetylcysteine in Amyotrophic Lateral Sclerosis. Arch. Neurol. 1995;52:559–564. doi: 10.1001/archneur.1995.00540300031009. [PubMed] [CrossRef] [Google Scholar]
84. Obrador E., Salvador R., Marchio P., López-Blanch R., Jihad-Jebbar A., Rivera P., Vallés S.L., Banacloche S., Alcácer J., Colomer N., et al. Nicotinamide Riboside and Pterostilbene Cooperatively Delay Motor Neuron Failure in ALS SOD1G93A Mice. Mol. Neurobiol. 2021;58:1345–1371. doi: 10.1007/s12035-020-02188-7. [PubMed] [CrossRef] [Google Scholar]
85. de la Rubia J.E., Drehmer E., Platero J.L., Benlloch M., Caplliure-Llopis J., Villaron-Casales C., de Bernardo N., AlarcÓn J., Fuente C., Carrera S., et al. Efficacy and Tolerability of EH301 for Amyotrophic Lateral Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Human Pilot Study. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2019;20:1–8. doi: 10.1080/21678421.2018.1536152. [PubMed] [CrossRef] [Google Scholar]
86. Kirsch M., De Groot H. NAD(P)H, a Directly Operating Antioxidant? FASEB J. 2001;15:1569–1574. doi: 10.1096/fj.00-0823hyp. [PubMed] [CrossRef] [Google Scholar]
87. Vermot A., Petit-Härtlein I., Smith S.M.E., Fieschi F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants. 2021;10:890. doi: 10.3390/antiox10060890. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
88. Moosmann B., Behl C. Antioxidants as Treatment for Neurodegenerative Disorders. Expert Opin. Investig. Drugs. 2002;11:1407–1435. doi: 10.1517/13543784.11.10.1407. [PubMed] [CrossRef] [Google Scholar]
89. Wang J.-Y., Wen L.-L., Huang Y.-N., Chen Y.-T., Ku M.-C. Dual Effects of Antioxidants in Neurodegeneration: Direct Neuroprotection against Oxidative Stress and Indirect Protection via Suppression of Glia-Mediated Inflammation. Curr. Pharm. Des. 2006;12:3521–3533. doi: 10.2174/138161206778343109. [PubMed] [CrossRef] [Google Scholar]
90. Robledinos-Antón N., Fernández-Ginés R., Manda G., Cuadrado A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019;2019:9372182. doi: 10.1155/2019/9372182. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
91. Granucci E.J., Griciuc A., Mueller K.A., Mills A.N., Le H., Dios A.M., McGinty D., Pereira J., Elmaleh D., Berry J.D., et al. Cromolyn Sodium Delays Disease Onset and Is Neuroprotective in the SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis. Sci. Rep. 2019;9:17728. doi: 10.1038/s41598-019-53982-w. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
92. Bhat A., Ray B., Mahalakshmi A.M., Tuladhar S., Nandakumar D.N., Srinivasan M., Essa M.M., Chidambaram S.B., Guillemin G.J., Sakharkar M.K. Phosphodiesterase-4 Enzyme as a Therapeutic Target in Neurological Disorders. Pharmacol. Res. 2020;160:105078. doi: 10.1016/j.phrs.2020.105078. [PubMed] [CrossRef] [Google Scholar]
93. Tefera T.W., Steyn F.J., Ngo S.T., Borges K. CNS Glucose Metabolism in Amyotrophic Lateral Sclerosis: A Therapeutic Target? Cell Biosci. 2021;11:14. doi: 10.1186/s13578-020-00511-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
94. Veldink J.H., Kalmijn S., Groeneveld G.-J., Wunderink W., Koster A., de Vries J.H.M., van der Luyt J., Wokke J.H.J., Van den Berg L.H. Intake of Polyunsaturated Fatty Acids and Vitamin E Reduces the Risk of Developing Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry. 2007;78:367–371. doi: 10.1136/jnnp.2005.083378. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
95. Dewsbury L.S., Lim C.K., Steiner G.Z. The Efficacy of Ketogenic Therapies in the Clinical Management of People with Neurodegenerative Disease: A Systematic Review. Adv. Nutr. 2021;12:1571–1593. doi: 10.1093/advances/nmaa180. [PMC free article] [PubMed] [CrossRef] [Google Scholar]

